Search
- Page Path
- HOME > Search
Brief Reports
- Safety monitoring of COVID-19 vaccination among adolescents aged 12 to 17 years old in the Republic of Korea
- Seontae Kim, Insob Hwang, Mijeong Ko, Yunhyung Kwon, Yeon-Kyeng Lee
- Osong Public Health Res Perspect. 2022;13(3):230-237. Published online June 10, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0122
- 4,145 View
- 139 Download
- 6 Web of Science
- 7 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
This study aimed to disseminate information on coronavirus disease 2019 (COVID-19) vaccine safety among adolescents aged 12 to 17 years in the Republic of Korea. Methods: Two databases were used to assess COVID-19 vaccine safety in adolescents aged 12 to 17 years who completed the primary Pfizer-BioNTech vaccination series. Adverse events reported to the web-based COVID-19 vaccination management system (CVMS) and collected in the text message-based system were analyzed. Results: From March 5, 2021 to February 13, 2022, 12,216 adverse events among 12- to 17-yearolds were reported to the CVMS, of which 97.1% were non-serious adverse events and 2.9% were serious adverse events, including 85 suspected cases of anaphylaxis, 74 suspected cases of myocarditis and/or pericarditis, and 2 deaths. From December 13, 2021 to January 26, 2022, 10,389 adolescents responded to a text message survey, and local/systemic adverse events were more common after dose 2 than after dose 1. The most commonly reported events following either vaccine dose were pain at the injection site, headache, fatigue/tiredness, and myalgia. Conclusion: The overall results are consistent with previous findings; the great majority of adverse events were non-serious, and serious adverse events were rare among adolescents aged 12 to 17 years following Pfizer-BioNTech COVID-19 vaccination. -
Citations
Citations to this article as recorded by- Suspected Myocarditis after mRNA COVID-19 Vaccination among South Korean Adolescents
Mi Jin Kim, Jin Hee Kim, Hyun Ok Jun, Kyung Min Kim, Min Sub Jeung, Jun Sung Park
Journal of Pediatric Infectious Diseases.2024; 19(02): 075. CrossRef - Characterization of Brighton Collaboration criteria for myocarditis and pericarditis following COVID-19 vaccine in Korean adolescents
Jue Seong Lee, HyoSug Choi, Seung Hwan Shin, Myung-Jae Hwang, Sara Na, Jong Hee Kim, Sangshin Park, Yoonsun Yoon, Hyun Mi Kang, Bin Ahn, Kyoungsan Seo, Young June Choe
Vaccine.2024;[Epub] CrossRef - Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years: an updated systematic review and meta-analysis
Peng Gao, Liang-Yu Kang, Jue Liu, Min Liu
World Journal of Pediatrics.2023; 19(11): 1041. CrossRef - Incidence of myopericarditis after mRNA COVID-19 vaccination: A meta-analysis with focus on adolescents aged 12–17 years
Bao-Qiang Guo, Hong-Bin Li, Li-Qiang Yang
Vaccine.2023; 41(28): 4067. CrossRef - Safety monitoring of COVID-19 vaccines: February 26, 2021, To June 4, 2022, Republic of Korea
Yeon-Kyeng Lee, Yunhyung Kwon, Yesul Heo, Eun Kyoung Kim, Seung Yun Kim, Hoon Cho, Seontae Kim, Mijeong Ko, Dosang Lim, Soon-Young Seo, Enhi Cho
Clinical and Experimental Pediatrics.2023; 66(10): 415. CrossRef - Risk of Coronavirus Disease 2019 Messenger RNA Vaccination-Associated Myocarditis and Pericarditis – A Systematic Review of Population-Based Data
Yen-Ching Lin, Chia-Hsuin Chang, Wei-Ju Su, Chin-Hui Yang, Jann-Tay Wang
Risk Management and Healthcare Policy.2023; Volume 16: 2085. CrossRef - COVID-19 Vaccination in Korea: Past, Present, and the Way Forward
Eliel Nham, Joon Young Song, Ji Yun Noh, Hee Jin Cheong, Woo Joo Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef
- Suspected Myocarditis after mRNA COVID-19 Vaccination among South Korean Adolescents
- COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021
- Insob Hwang, Kyeongeun Park, Tae Eun Kim, Yunhyung Kwon, Yeon-Kyeng Lee
- Osong Public Health Res Perspect. 2021;12(6):396-402. Published online December 21, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0310
- 7,228 View
- 191 Download
- 13 Web of Science
- 11 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material 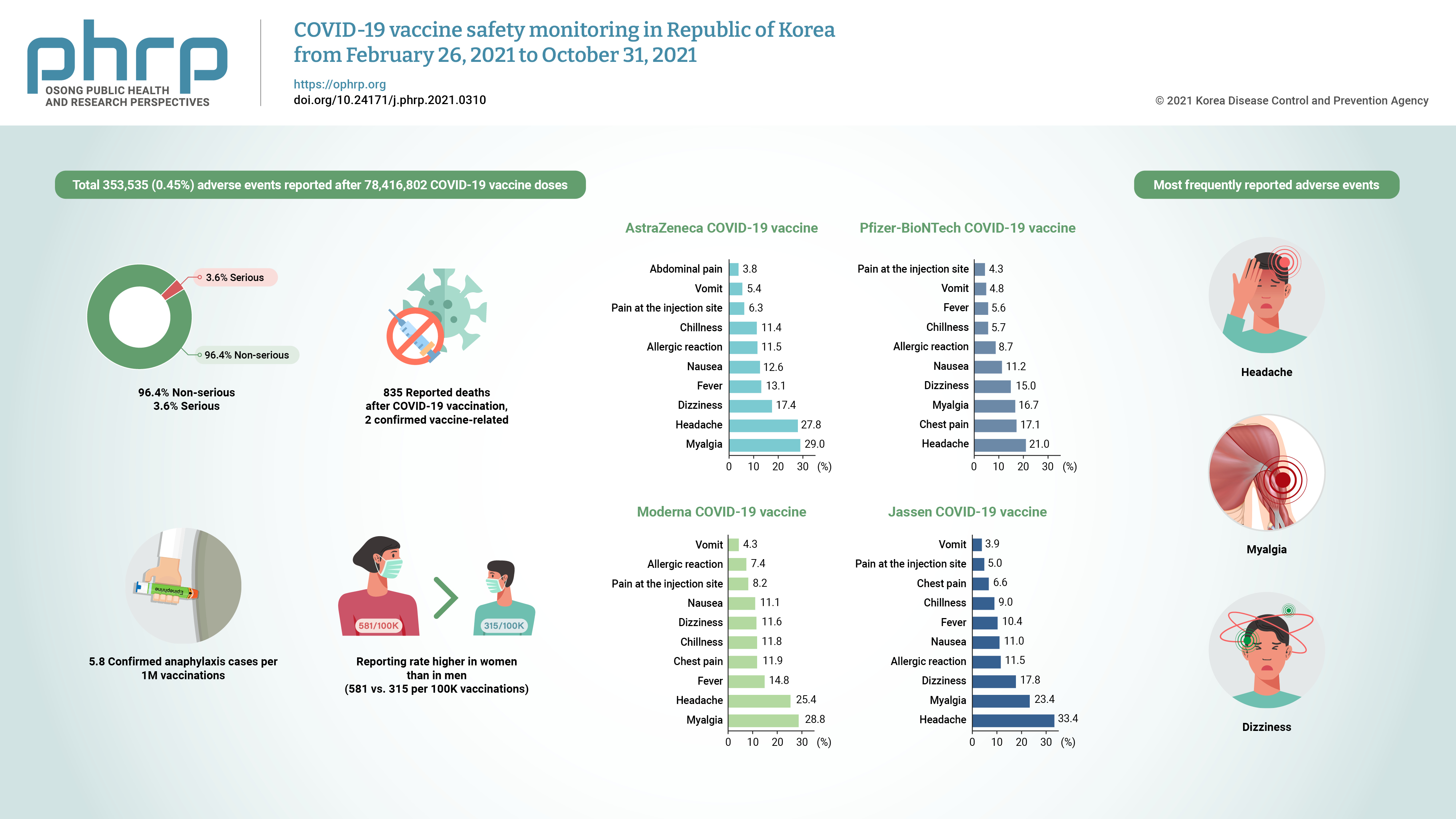
- Objectives
This study aimed to present data on reported adverse events following coronavirus disease 2019 (COVID-19) vaccination in Republic of Korea from February 26 to October 31, 2021, and to determine whether any significant patterns emerged from an analysis of the characteristics of suspected adverse event cases for each type of vaccine.
Methods
Adverse events following COVID-19 vaccination reported by medical doctors and forensic pathologists were analyzed. Cases of suspected anaphylaxis were classified using the Brighton Collaboration definition.
Results
By October 31, 2021, a total of 353,535 (0.45%) adverse events were reported after 78,416,802 COVID-19 vaccine doses. Of the adverse events, 96.4% were non-serious and 3.6% were serious. The most frequently reported adverse events were headache, myalgia, and dizziness. Of the 835 reported deaths after COVID-19 vaccination, 2 vaccine-related deaths were confirmed. Suspected anaphylaxis was confirmed in 454 cases using the Brighton Collaboration definition.
Conclusion
The commonly reported symptoms were similar to those described in clinical trials. Most reported adverse events were non-serious, and the reporting rate of adverse events following COVID-19 vaccination was higher in women than in men (581 vs. 315 per 100,000 vaccinations). Confirmed anaphylaxis was reported in 5.8 cases per 1,000,000 vaccinations. -
Citations
Citations to this article as recorded by- A Nationwide Survey of mRNA COVID-19 Vaccinee’s Experiences on Adverse Events and Its Associated Factors
Dongwon Yoon, Ha-Lim Jeon, Yunha Noh, Young June Choe, Seung-Ah Choe, Jaehun Jung, Ju-Young Shin
Journal of Korean Medical Science.2023;[Epub] CrossRef - Temporal association between the age-specific incidence of Guillain-Barré syndrome and SARS-CoV-2 vaccination in Republic of Korea: a nationwide time-series correlation study
Hyunju Lee, Donghyok Kwon, Seoncheol Park, Seung Ri Park, Darda Chung, Jongmok Ha
Osong Public Health and Research Perspectives.2023; 14(3): 224. CrossRef - Safety monitoring of COVID-19 vaccines: February 26, 2021, To June 4, 2022, Republic of Korea
Yeon-Kyeng Lee, Yunhyung Kwon, Yesul Heo, Eun Kyoung Kim, Seung Yun Kim, Hoon Cho, Seontae Kim, Mijeong Ko, Dosang Lim, Soon-Young Seo, Enhi Cho
Clinical and Experimental Pediatrics.2023; 66(10): 415. CrossRef - Allergic Reactions to COVID-19 Vaccines: Risk Factors, Frequency, Mechanisms and Management
Nicoletta Luxi, Alexia Giovanazzi, Alessandra Arcolaci, Patrizia Bonadonna, Maria Angiola Crivellaro, Paola Maria Cutroneo, Carmen Ferrajolo, Fabiana Furci, Lucia Guidolin, Ugo Moretti, Elisa Olivieri, Giuliana Petrelli, Giovanna Zanoni, Gianenrico Senna,
BioDrugs.2022; 36(4): 443. CrossRef - Safety monitoring of COVID-19 vaccination among adolescents aged 12 to 17 years old in the Republic of Korea
Seontae Kim, Insob Hwang, Mijeong Ko, Yunhyung Kwon, Yeon-Kyeng Lee
Osong Public Health and Research Perspectives.2022; 13(3): 230. CrossRef - Incidence and Characteristics of Adverse Events after COVID-19 Vaccination in a Population-Based Programme
Laura Bonzano, Olivera Djuric, Pamela Mancuso, Lidia Fares, Raffaele Brancaccio, Marta Ottone, Eufemia Bisaccia, Massimo Vicentini, Alessia Cocconcelli, Alfonso Motolese, Rostyslav Boyko, Paolo Giorgi Rossi, Alberico Motolese
Vaccines.2022; 10(7): 1111. CrossRef - Global Predictors of COVID-19 Vaccine Hesitancy: A Systematic Review
Carla Pires
Vaccines.2022; 10(8): 1349. CrossRef - Anaphylaxis and Related Events Following COVID‐19 Vaccination: A Systematic Review
Pradipta Paul, Emmad Janjua, Mai AlSubaie, Vinutha Ramadorai, Beshr Mushannen, Ahamed Lazim Vattoth, Wafa Khan, Khalifa Bshesh, Areej Nauman, Ibrahim Mohammed, Imane Bouhali, Mohammed Khalid, Dalia Zakaria
The Journal of Clinical Pharmacology.2022; 62(11): 1335. CrossRef - Adverse events of the Pfizer-BioNTech COVID-19 vaccine in Korean children and adolescents aged 5 to 17 years
Seontae Kim, Yeseul Heo, Soon-Young Seo, Do Sang Lim, Enhi Cho, Yeon-Kyeng Lee
Osong Public Health and Research Perspectives.2022; 13(5): 382. CrossRef - COVID-19 Vaccination in Korea: Past, Present, and the Way Forward
Eliel Nham, Joon Young Song, Ji Yun Noh, Hee Jin Cheong, Woo Joo Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Self-reported adverse events after 2 doses of COVID-19 vaccine in Korea
Yunhyung Kwon, Insob Hwang, Mijeong Ko, Hyungjun Kim, Seontae Kim, Soon-Young Seo, Enhi Cho, Yeon-Kyeng Lee
Epidemiology and Health.2022; 45: e2023006. CrossRef
- A Nationwide Survey of mRNA COVID-19 Vaccinee’s Experiences on Adverse Events and Its Associated Factors
Original Articles
- Delays in the diagnosis and treatment of tuberculosis during the COVID-19 outbreak in the Republic of Korea in 2020
- Jiyeon Yang, Yunhyung Kwon, Jaetae Kim, Yoojin Jang, Jiyeon Han, Daae Kim, Hyeran Jeong, Hyekyung Park, Eunhye Shim
- Osong Public Health Res Perspect. 2021;12(5):293-303. Published online September 23, 2021
- DOI: https://doi.org/10.24171/j.phrp.2021.0063
- 7,389 View
- 188 Download
- 8 Web of Science
- 8 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 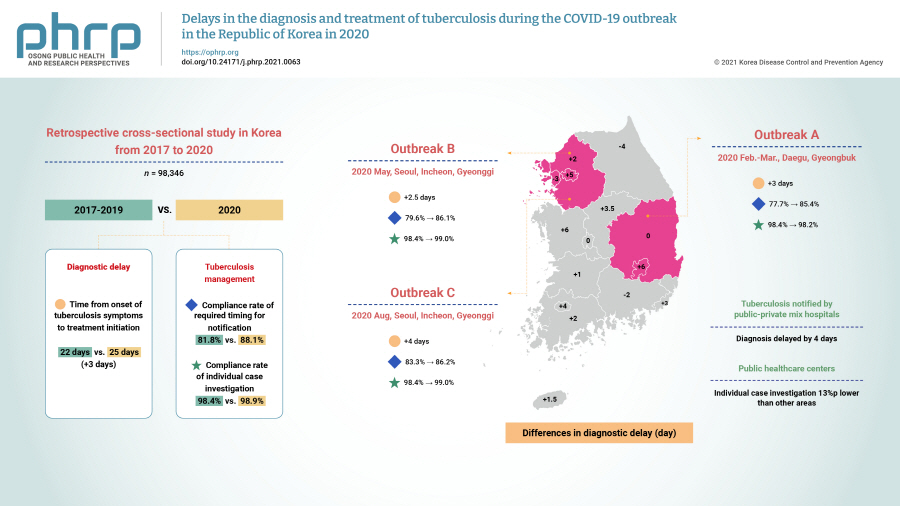
- Objectives
We investigated the impact of the coronavirus disease 2019 (COVID-19) pandemic on tuberculosis (TB) management in the Republic of Korea (ROK).
Methods
This retrospective cross-sectional study used nationwide ROK TB notification data (98,346 cases) from 2017 to 2020. The median time from the onset of TB symptoms to treatment initiation and the compliance rates with the required timing for notification and individual case investigations were measured and compared across periods and regions affected by the COVID-19 epidemic.
Results
TB diagnosis during the COVID-19 pandemic was delayed. The median time to TB treatment initiation (25 days) in 2020 increased by 3 days compared to that of the previous 3 years (22 days) (p<0.0001). In the outbreak in Seoul, Incheon, and Gyeonggi province during August, the time to TB diagnosis was 4 days longer than in the previous 3 years (p=0.0303). In the outbreak in Daegu and Gyeongbuk province from February to March 2020, the compliance rate with the required timing for individual case investigations was 2.2%p points lower than in other areas in 2020 (p=0.0148). For public health centers, the rate was 13%p lower than in other areas (80.3% vs. 93.3%, p=0.0003).
Conclusion
TB diagnoses during the COVID-19 pandemic in the ROK were delayed nationwide, especially for patients notified by public-private mix TB control hospitals. TB individual case investigations were delayed in regional COVID-19 outbreak areas (Daegu and Gyeongbuk province), especially in public health centers. Developing strategies to address this issue will be helpful for sustainable TB management during future outbreaks. -
Citations
Citations to this article as recorded by- A Review of the Impact of Patent Medicine Vendors in Driving Community Tuberculosis Case Finding in the COVID-19 Pandemic in Nigeria
Arinze Emmanuel Ajogwu, Onwubiko Iheanyichukwu Samuel, Nnanyelugo Longinus Ochike, Uzoma Chidinma Ajegbo, Chinedu Paschal Maduka
Matrix Science Medica.2024; 8(2): 33. CrossRef - Tuberculosis: Republic of Korea, 2021
Jinsoo Min, Hyung Woo Kim, Ju Sang Kim
Tuberculosis and Respiratory Diseases.2023; 86(1): 67. CrossRef - Prevalence and associated factors of diabetes mellitus among patients with tuberculosis in South Korea from 2011 to 2018: a nationwide cohort study
Dawoon Jeong, Jeongha Mok, Doosoo Jeon, Hee-Yeon Kang, Hee Jin Kim, Hee-Sun Kim, Jeong Mi Seo, Hongjo Choi, Young Ae Kang
BMJ Open.2023; 13(3): e069642. CrossRef - Increased Healthcare Delays in Tuberculosis Patients During the First Wave of COVID-19 Pandemic in Korea: A Nationwide Cross-Sectional Study
Jinsoo Min, Yousang Ko, Hyung Woo Kim, Hyeon-Kyoung Koo, Jee Youn Oh, Yun-Jeong Jeong, Hyeon Hui Kang, Kwang Joo Park, Yong Il Hwang, Jin Woo Kim, Joong Hyun Ahn, Yangjin Jegal, Ji Young Kang, Sung-Soon Lee, Jae Seuk Park, Ju Sang Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Time trend prediction and spatial–temporal analysis of multidrug-resistant tuberculosis in Guizhou Province, China, during 2014–2020
Wang Yun, Chen Huijuan, Liao Long, Lu Xiaolong, Zhang Aihua
BMC Infectious Diseases.2022;[Epub] CrossRef - Real-world association of adherence with outcomes and economic burden in patients with tuberculosis from South Korea claims data
Sun-Hong Kwon, Jin Hyun Nam, Hye-Lin Kim, Hae-Young Park, Jin-Won Kwon
Frontiers in Pharmacology.2022;[Epub] CrossRef - The Impact of the COVID-19 Pandemic on Tuberculosis Case Notification and Treatment Outcomes in Eswatini
Hloniphile Victory Masina, I-Feng Lin, Li-Yin Chien
International Journal of Public Health.2022;[Epub] CrossRef - Trends in incidences of newly notified tuberculosis in Jeju Province, Korea, 2017-2021
Jinhee Kim, Nam-Hun Kang, Jong-Myon Bae
Journal of Medicine and Life Science.2022; 19(3): 103. CrossRef
- A Review of the Impact of Patent Medicine Vendors in Driving Community Tuberculosis Case Finding in the COVID-19 Pandemic in Nigeria
- Results of Tuberculosis Contact Investigation in Congregate Settings in Korea, 2013
- Yunhyung Kwon, So Jung Kim, Jieun Kim, Seol-yi Kim, Eun Mi Song, Eun Jung Lee, Yun Choi, Yejin Kim, Byoung ok Lim, Da Sul Kim, Duksun Choi, Hye Sung Kim, Ji Eun Park, Ji-eun Yun, Jin A. Park, Jong Rak Jung, Joo-kyoung Kim, Sang Hee Kang, Seo Yean Hong, Seung Jae Lee, Soo Jin Park, Sun Hwa Park, Sunhye Yoon, Yoonsun Kim, Yunjeong Choi, Yun Jeong Seo, Yul A Seo, Jiseon Park, Minhee Sung, Minjang Shin, Hyunjin Son, Yeonkyeng Lee, Unyeong Go, Geun-Yong Kwon
- Osong Public Health Res Perspect. 2014;5(Suppl):S30-S36. Published online December 31, 2014
- DOI: https://doi.org/10.1016/j.phrp.2014.10.010
- 3,409 View
- 22 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF - Objectives
This study aimed to check the status of the contact investigation in congregate settings to eradicate tuberculosis (TB) in the Republic of Korea.
Methods
The “Integrated System for Disease and Public Health Management” is used for care and follow-up for patients and contacts of TB. We downloaded data for contact investigations conducted from January to December 2013.
Results
A total of 1,200 contact investigations in congregate settings were carried out by 25 field investigators in 2013. We performed the status of contact investigation, TB, and LTBI rate by age, accept rate of LTBI treatment, and complete rate of LTBI treatment during 2013. A total of 1,547 index TB patients, 149,166 contacts, and 259 additional TB patients were found through the investigation. Kindergartens showed the highest LTBI rate, 19.8%, among educational facilities. The second highest was in elementary schools and the subtotal LTBI rate of educational facilities was 7.8%. Social welfare/correctional facilities and workplaces showed relatively high LTBI rates of 23.8% and 23.6%, respectively. By age, individuals >35 years showed the highest LTBI rate, followed by those aged 0–4 years, 30–34 years, and 5–9 years, with rates of 18.1%, 16.4%, and 15.4% respectively. When comparing the tuberculin skin test (TST) positive conversion ratio by facility, middle school and high school were relatively high compared to the others. The accept rate of LTBI treatment in the workplace was lowest at 63% and the complete rate in elementary schools was lowest at 76.5%.
Conclusion
TB contact investigation is considered as a meaningful strategy for preventing TB outbreaks in congregate settings and decreasing the prevalence of TB in young people. Results of this study could be used to establish the LTBI management policy. -
Citations
Citations to this article as recorded by- Latent Tuberculosis Cascade of Care Among Healthcare Workers: A Nationwide Cohort Analysis in Korea Between 2017 and 2018
Jinsoo Min, Hyung Woo Kim, Joon Young Choi, Ah Young Shin, Ji Young Kang, Yunhee Lee, Jun-Pyo Myong, Hyunsuk Jeong, Sanghyuk Bae, Hyeon-Kyoung Koo, Sung-Soon Lee, Jae Seuk Park, Hyeon Woo Yim, Ju Sang Kim
Journal of Korean Medical Science.2022;[Epub] CrossRef - Risk of active tuberculosis development in contacts exposed to infectious tuberculosis in congregate settings in Korea
Shin Young Park, Sunmi Han, Young-Man Kim, Jieun Kim, Sodam Lee, Jiyeon Yang, Un-Na Kim, Mi-sun Park
Scientific Reports.2020;[Epub] CrossRef - The risk of active tuberculosis among individuals living in tuberculosis-affected households in the Republic of Korea, 2015
Jiyeon Yang, Sodam Lee, Suhyeon Oh, Sunmi Han, Shin Young Park, Youngman Kim, Jieun Kim, Mi-sun Park, Philip C. Hill
PLOS ONE.2019; 14(12): e0225744. CrossRef - The Infectivity of Pulmonary Tuberculosis in Korean Army Units: Evidence from Outbreak Investigations
Chang-gyo Yoon, Dong Yoon Kang, Jaehun Jung, Soo Yon Oh, Jin Beom Lee, Mi-Hyun Kim, Younsuk Seo, Hee-Jin Kim
Tuberculosis and Respiratory Diseases.2019; 82(4): 298. CrossRef - Tuberculosis prevention and care in Korea: Evolution of policy and practice
Unyeong Go, Misun Park, Un-Na Kim, Sodam Lee, Sunmi Han, Joosun Lee, Jiyeon Yang, Jieun Kim, Shinyoung Park, Youngman Kim, Hyosoon Yoo, Jeongok Cha, Wonseo Park, Haeyoung Kang, Hwon Kim, Guri Park, Minjung Kim, Ok Park, Hyunjin Son, Enhi Cho, Kyoungin Na,
Journal of Clinical Tuberculosis and Other Mycobac.2018; 11: 28. CrossRef - The Prevalence and Risk Factors of Latent Tuberculosis Infection among Health Care Workers Working in a Tertiary Hospital in South Korea
Jae Seuk Park
Tuberculosis and Respiratory Diseases.2018; 81(4): 274. CrossRef
- Latent Tuberculosis Cascade of Care Among Healthcare Workers: A Nationwide Cohort Analysis in Korea Between 2017 and 2018



 First
First Prev
Prev


